Why Alberta for your next clinical trial?

Every year, pharmaceutical, biotech, and medical device companies from across the globe leverage the advantages of Alberta’s unique clinical trial environment to advance their research.
That’s because our province is home to world-class investigators, a high-quality clinical trial infrastructure and one of the largest integrated health systems in North America. Our effective ethics framework allows trials to start faster and with less operational burden. And our steadfast focus on continuous improvement ensures industry partners benefit from cutting-edge research capabilities at every stage.

More organizations are looking to Alberta to advance their clinical trials. Discover what sets us apart.
About Alberta
Alberta is Canada’s fourth most populated province – home to more than four million people all served by one integrated health care system. While primarily English-speaking, the population is distinctly diverse, with representation from all ethnic groups and almost 25% of residents belonging to a underrepresented minority group.
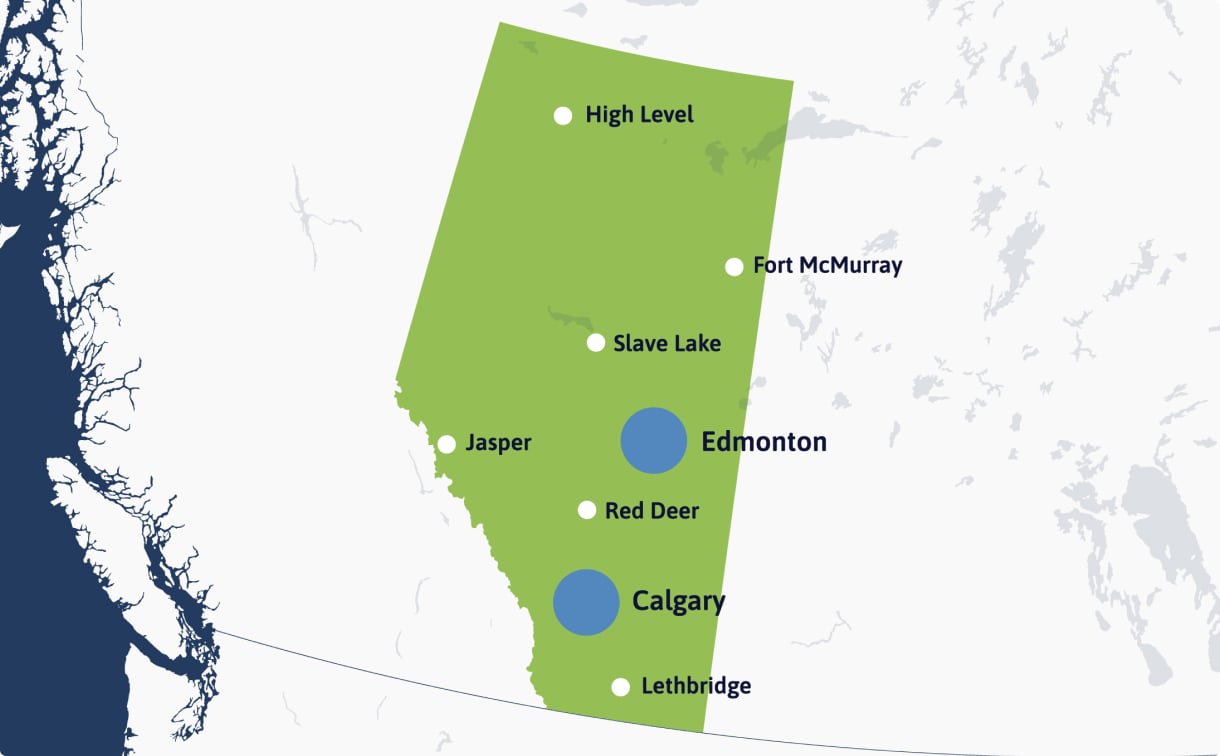
More than 70 per cent of Albertans live in an urban area, the majority within the Calgary–Edmonton corridor (one of the most densely populated areas in Canada). This positions much of the population close to major hospitals, research centres, sites and academic institutes.
A Dynamic Clinical Trial Environment
With world-class universities, cutting-edge hospitals, and pioneering companies, Alberta is a top Canadian destination for all clinical trials, thriving across all major therapeutic areas. Alberta is also recognized as a global leader in leading and managing biotech clinical trials. Driven by our unique infrastructure and sector expertise, hundreds of biotech partners are currently engaged in trials across a range of therapeutic areas including oncology, infectious diseases, rare diseases, and genetic disorders.

3rd most active province for clinical trials
1100+ active clinical trials on clinicaltrials.gov in Alberta
2 of Canada's most active clinical research cities
Edmonton and Calgary are two of Canada’s most active clinical research cities, with cutting-edge facilities, top-tier institutions, and a thriving network of healthcare professionals driving significant advancements in health research.
Skilled research workforce
Alberta’s life sciences industry employs over 15,000 highly trained professionals, including research coordinators, nurses, and others, many with advanced degrees.
High economic impact
$1 Billion Revenue generated by Alberta’s life sciences sector.
Diverse Range of Trials
From early phase studies requiring bioanalytical support, pharmacokinetic modeling and simulation to phase IV studies involving outpatient facilities and real-world evidence.
Open trial phases
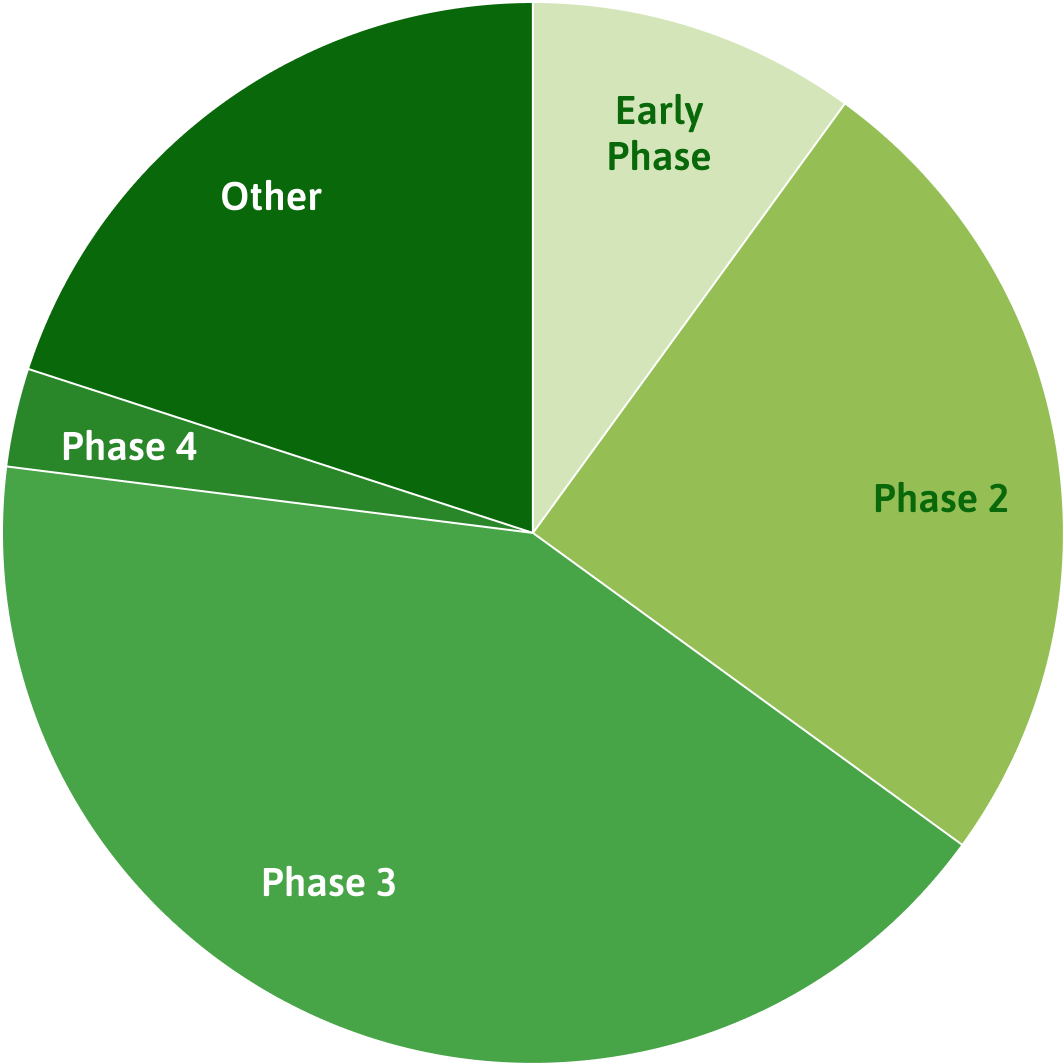
Source: ClinicalTrials.gov. Last updated February 2021.
Top therapeutic areas by study volume
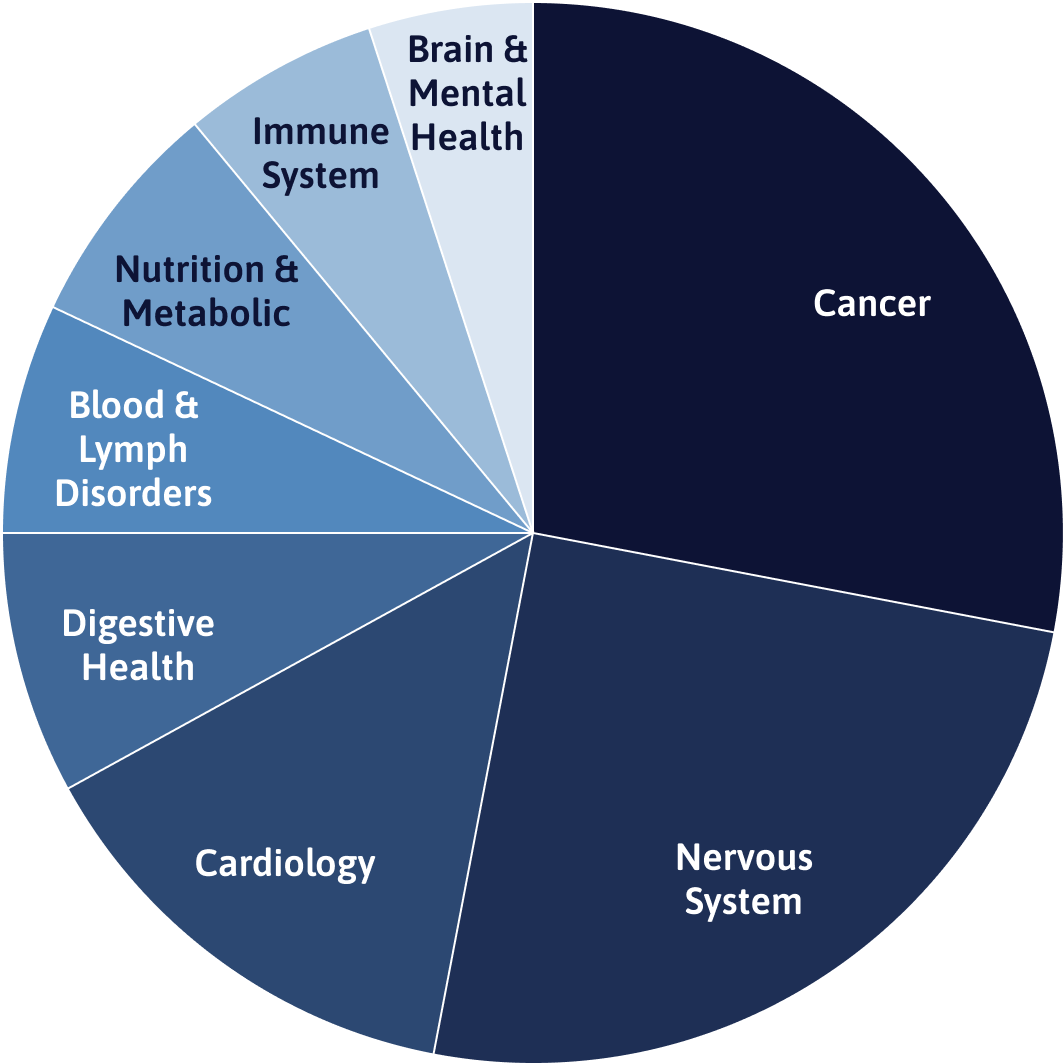
Source: ClinicalTrials.gov. Last updated February 2021.
Expertise across a range of therapeutic areas
Alberta is home to some of the world’s most respected thought leaders and world-renowned institutes across a range of therapeutic areas including cardiovascular health, oncology, rare diseases, and nervous system disorders. Our province’s investigators are leading clinicians and experienced, highly trained clinical trialists.
World-Class Infrastructure
Housed within our collaborative network of academic institutions (including two of Canada’s leading research universities), hospitals and medical research centres is an extensive range of cutting-edge labs and facilities. Our province is home to a full spectrum of technologies including advanced imaging, biobanking, bioanalysis and real-life simulation.

State-of-the art Phase I Clinical Trial Units
High-Quality Patient Care Areas
Modern Research Laboratories
Specialized Treatment Facilities
Cutting-Edge Hospitals and Major Cancer Centers
High-Tech Imaging and Diagnostics
Streamlined Access to High-Quality Data
Alberta’s public health system – overseen by Alberta Health Services – is the largest integrated healthcare system in Canada and the fifth largest in the world. This unified system allows for streamlined access to high quality, robust patient data. A single electronic medical records system covers the majority of the patient population. Alberta is home to one of the most robust, integrated and accessible health data repositories in the world. Our data assets allow for streamlined access to high quality data to support comprehensive feasibility studies, regulatory monitoring, and clinical trials across a population of over 4 million.
Single EMR System for a 4.6m population
Data linkage and analytics platforms
Real-world evidence capabilities
Robust data repositories and registries
Optimized Participant Recruitment
Patients and healthy participants suitable for clinical trials can be easily identified and engaged with. A single point-of-entry helps identify potential participants and enables contact with physicians, hospitals and research centres across the province. Key public awareness campaigns, such as Be the Cure, contribute to higher levels of clinical trial participation.

4.6m population from diverse backgrounds
Public Engagement Programs
Ecosystem Partners Recruitment Supports and Platforms
Choose Alberta for your clinical trial
If you’re considering bringing your clinical trial to Alberta or would like to learn more about our capabilities, we’re here to help.
