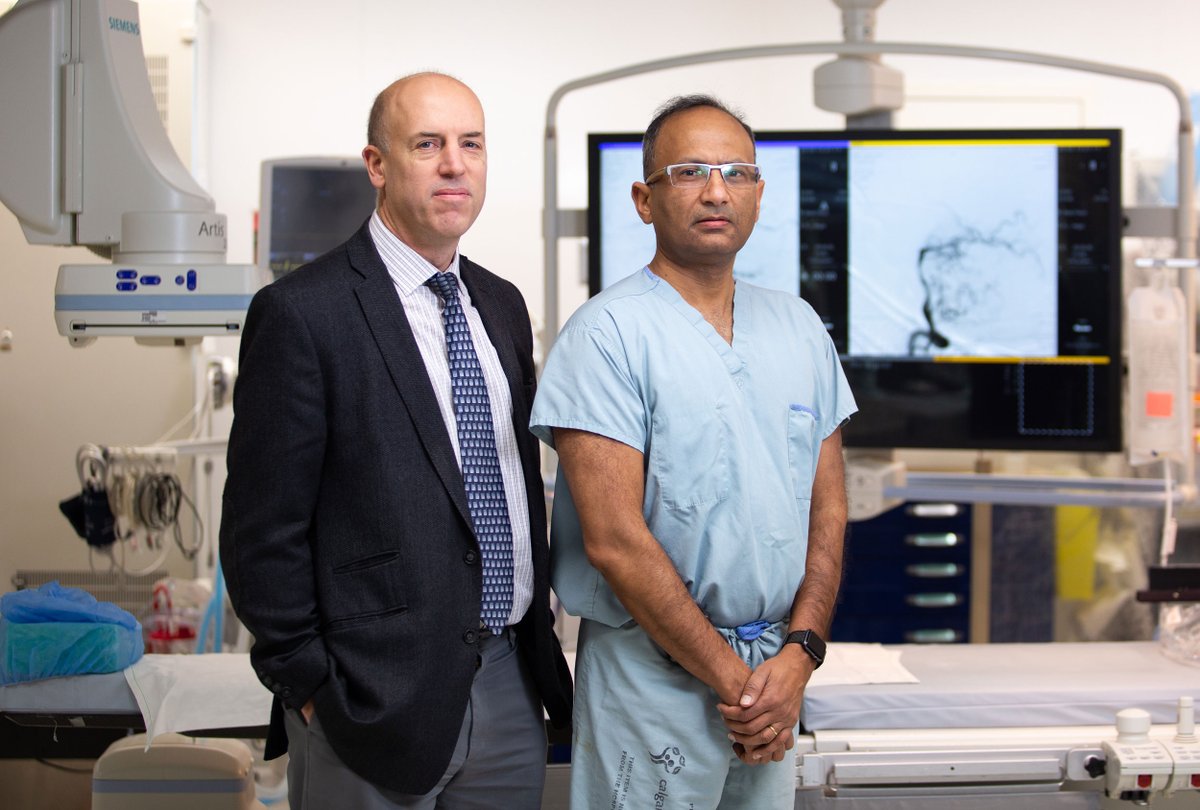
In 2016, Alberta researchers conducted a groundbreaking study that changed the standard of care for Ischemic Stroke worldwide. Through an innovative, international multi-site clinical trial, Dr. Michael Hill and his team from the University of Calgary successfully demonstrated the life-changing benefits of a new procedure: ESCAPE (Endovascular treatment for Small Core and Anterior circulation Proximal occlusion with Emphasis on minimizing CT to recanalization times).
Ischemic Stroke is caused when blood flow, through the artery that supplies oxygen-rich blood to the brain, becomes blocked. Typically, Ischemic Stroke patients have complications and suffer poor health outcomes due to permanent damage to brain tissue.
“Unfortunately, these patients can suffer from debilitating damage that seriously impacts their quality of life and future prognosis” says Dr. Hill, one of the lead researchers with the IMPACT Trial.
The ESCAPE Trial utilized a new procedure involving a thin tube, inserted through an artery, to remove clots in the brain. This new treatment showed tremendous benefits – including a decreased mortality rate and higher quality of care for post-stroke patients. The impact of the ESCAPE study was profound. It has resulted in a change in the standard of care for Ischemic Stroke worldwide and has improved the lives of countless patients.
“The impact to patients has been huge” adds Dr. Hill.
The ESCAPE trial clearly demonstrated the level of expertise present in Alberta – namely within stroke research. “The ESCAPE trial put Alberta on the map in the stroke community” says Dr. Hill, “they know what we can do, they know what we have done, they know what is possible.”
The study, which was co-led by The University of Calgary and The University of Alberta, brought together Alberta’s leading research institutions to utilize a range of expertise while showcasing another key capability of provincial research assets: the ability to lead and manage large, international multi-site trials.
“We had access to people who could translate and support a global, multi-cultural trial.” Says Dr. Michael Hill. “We were able to manage regulatory submissions, oversee and compile a large amount of data and offer specialized biostatistical and operational expertise.”
To address the challenges involved in managing this type of study, the ESCAPE team developed a data-management group, overseeing randomization and data management of the trial results from multiple sites. With any multi-national trial, ethical standards and specific regulatory requirements were different across countries. By leaning on the past experience of the research group, the ESCAPE team rose to the challenge and was able to seamlessly navigate these diverse requirements.”
Dr. Hill sees the ESCAPE trial as a shining example of what is possible in Alberta. “Our people were able to successfully execute this study at an incredibly high level – combining therapeutic area and operational expertise. This is something I am truly proud of.”
To learn more about the ESCAPE trial, visit https://cumming.ucalgary.ca/escape-stroke.