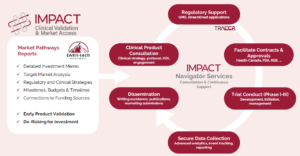
Bringing a medical device to market is no small feat. From navigating regulatory pathways to securing clinical validation, MedTech startups face a long and complex journey. That’s where the IMPACT Program comes in. A collaborative initiative between Innovate Calgary and the University of Calgary’s Cumming School of Medicine, IMPACT is designed to help MedTech companies accelerate their clinical trials and streamline the path to commercialization.
Created by Dr. Derek Exner, a Canada Research Chair (Tier 1) in Cardiovascular Clinical Trials and the inaugural Associate Dean of Innovation and Commercialization at the Cumming School of Medicine, IMPACT offers startups the expert guidance they need to navigate the intricacies of product development, regulatory approval, and clinical trials.
A Roadmap to Commercial Success
For startups looking to bring their innovations to market, IMPACT provides detailed commercialization roadmaps: offering clear, actionable steps to move forward. From quality management systems and preclinical testing to reimbursement strategies and post-market surveillance, the program equips companies with the tools they need to overcome regulatory hurdles and secure market access.
By integrating clinical and regulatory expertise early on, IMPACT helps MedTech startups de-risk their investments, optimize resources, and build strategic partnerships that are critical to commercial success.
Tailored Clinical Trial Support
One of the biggest challenges MedTech startups face is designing and executing cost-effective clinical trials. IMPACT simplifies this process by offering a tailored, integrated approach to clinical research. Through partnerships with the University of Calgary and a network of Alberta-based and pan-Canadian collaborators, IMPACT’s clinical and regulatory consultants work directly with startups to facilitate the rapid deployment of studies. This hands-on support ensures that clinical investigations are aligned with regulatory expectations, whether companies are:
- Gathering data for usability and product development
- Seeking initial market clearances from regulatory bodies
- Establishing novel claims on a previously approved device
- Building a case for reimbursement
Members of IMPACT work directly with the startup team to identify priorities and ensure that studies are designed and implemented in a way that supports multiple overlapping objectives, creating a robust, actionable dataset, avoiding the need for costly downstream duplication.
Speeding Up Cost-Effective Clinical Trials
MedTech innovation moves fast, and startups need the flexibility to adapt their clinical research strategies as market conditions and capital availability evolve. That’s why IMPACT operates as an extension of the startup’s team, working alongside them to co-develop protocols, regulatory applications, and study agreements.
By leveraging partnerships with regional innovation hubs and industry stakeholders, IMPACT ensures a seamless transition between development and clinical validation. This not only expedites the regulatory process but also strengthens a startup’s ability to attract funding and grant support.
For early feasibility and first-in-human trials, IMPACT prioritizes speed, affordability, and personalized study design, enabling MedTech startups to move from concept to clinical validation faster than ever before.
Scaling to Multi-Site Pivotal Trials
As startups progress to later clinical stages, larger trials with 100-400+ participants become necessary for validation. While still smaller than large-scale Phase III pharmaceutical trials, these pivotal device trials require multi-site investigations to confirm safety, efficacy, and patient diversity.
IMPACT supports startups in expanding from single-site pilots to multi-site pivotal studies, leveraging a network of collaborators across Canada to ensure smooth execution. By guiding companies through this critical scale-up phase, IMPACT helps secure the data needed for regulatory approvals and investor confidence.
The Alberta Advantage: A Hub for MedTech Innovation
Alberta has long been recognized as a leader in clinical research, offering world-class infrastructure, fast site-startup times, and high-quality trial execution. Organizations such as Alberta Innovates, Clinical Trials Alberta, Innovate Calgary, ADEPT & ACAD, A-MEDICO, W21C, API, CAMS, CIM-TAC, and AbSPORU (to name a few) play a crucial role in fostering an environment where MedTech companies can thrive.
By leveraging Alberta’s established research ecosystem, IMPACT provides startups with the resources and expertise they need to accelerate clinical development, reduce regulatory roadblocks, and enhance investor confidence.
Driving MedTech Forward
The journey from concept to market in the MedTech space is filled with challenges, but with the right support, startups can navigate these hurdles more efficiently. The IMPACT Program offers a unique blend of clinical, regulatory, and commercialization expertise, helping companies bring innovative products to market faster, with fewer risks, and greater investor confidence.
For MedTech entrepreneurs looking to take their innovations to the next level, IMPACT isn’t just a program; it’s a strategic partner in success.
For more information about IMPACT, visit IMPACT – Innovate Calgary.